This is a Four Basic Types of Chemical Reactions template that can be used in Chemistry class to present visually the reactions. The template is customizable with a few drags and drops in MyDraw.
Download Template:


Download Template:


Basic terminology
Reactants- are compounds that react to produce new ones.
Products- these are the result of newly formed compounds after interaction with other compounds.
Chemical equation- it is a mathematical statement that presents the formation of a product from the reactants.
Reaction rate- the speed index at which the reactants are transformed into products.
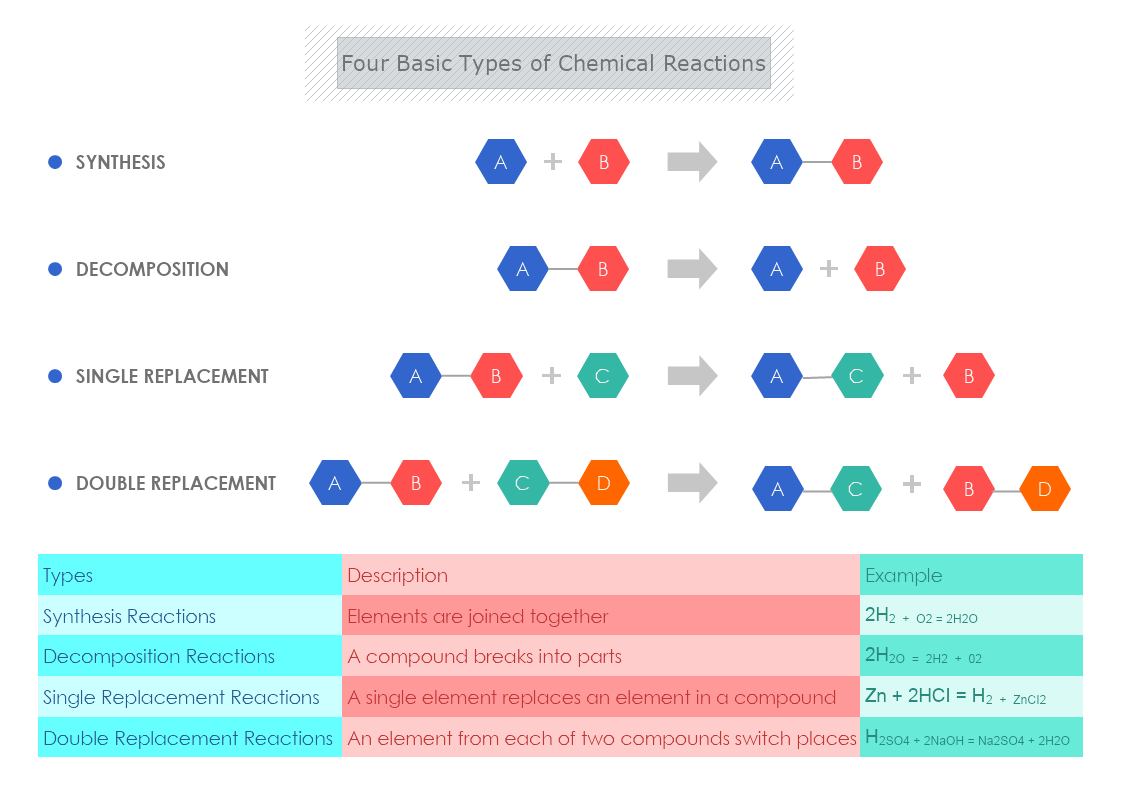
The Four Basic Types of Chemical Reactions with examples
Synthesis Reactions- in this type of reaction the elements are joined together.
- A + B = AB
- 2H2 + O2 = 2H2O
Decomposition Reactions- a compound breaks into parts.
- AB = A + B
- 2H2O = 2H2 + 02
Single Replacement Reactions- a single element replaces an element in a compound.
- AB + C = AC + B
- Zn + 2HCI = H2 + ZnCI2
Double Replacement Reactions An element from each of the two compounds switch places.
- AB + CD = AC + BD
- H2SO4 + 2NaOH = Na2SO4 + 2H2O
Interesting facts about chemical reactions
-
The molecule of substances remains the same, whereas the mixtures and the solutions are completely different from the chemical reactions.
- Chain of reaction is when a set of reactions happens due to one.
- The movement of the 3 small bones in the ear is caused by vibrations of the ear drum.
- More than 100,000 chemical reactions occur per second in our brains.
- The mixture of baking soda and vinegar produces carbon dioxide gas.
- The amount, quality, and mass remain the same in a chemical reaction.
How to create a template for the Four Basic Types of Chemical Reactions in MyDraw?
- Edit the ready-made template or open a “Blank Drawing” file to create your own.
- When you open a document in MyDraw a set of Basic and Connector shapes are loaded. You can choose from them and make the template.
- Also, from Library Gallery> ClipArt, you can select and browse through a variety of shapes you would like to use.
- From Library Gallery use the search library to find more shapes, suitable for your template.
- Once you have checked and marked the shapes, they will be loaded on the left side of your drawing panel.
- Drag and drop the shapes you would like to use into the drawing.
- Use the connector tools to arrange your diagram.
- To add fill, you can edit the Geometry Fill and Stroke from the Ribbon.
- In the Ribbon, you can select the Design tab to choose from a variety of shape styles and theme colors.
- Save the document in one of MyDraw’s native formats or export it in a preferred file format (PDF, SVG, EMF, VSDX, etc.).
- You can also export the document as a raster image.
