This is a Classes of Reactions template that is presented in a hierarchical chart. The template is customizable with a few drags and drops in MyDraw.
Download Template:


Download Template:


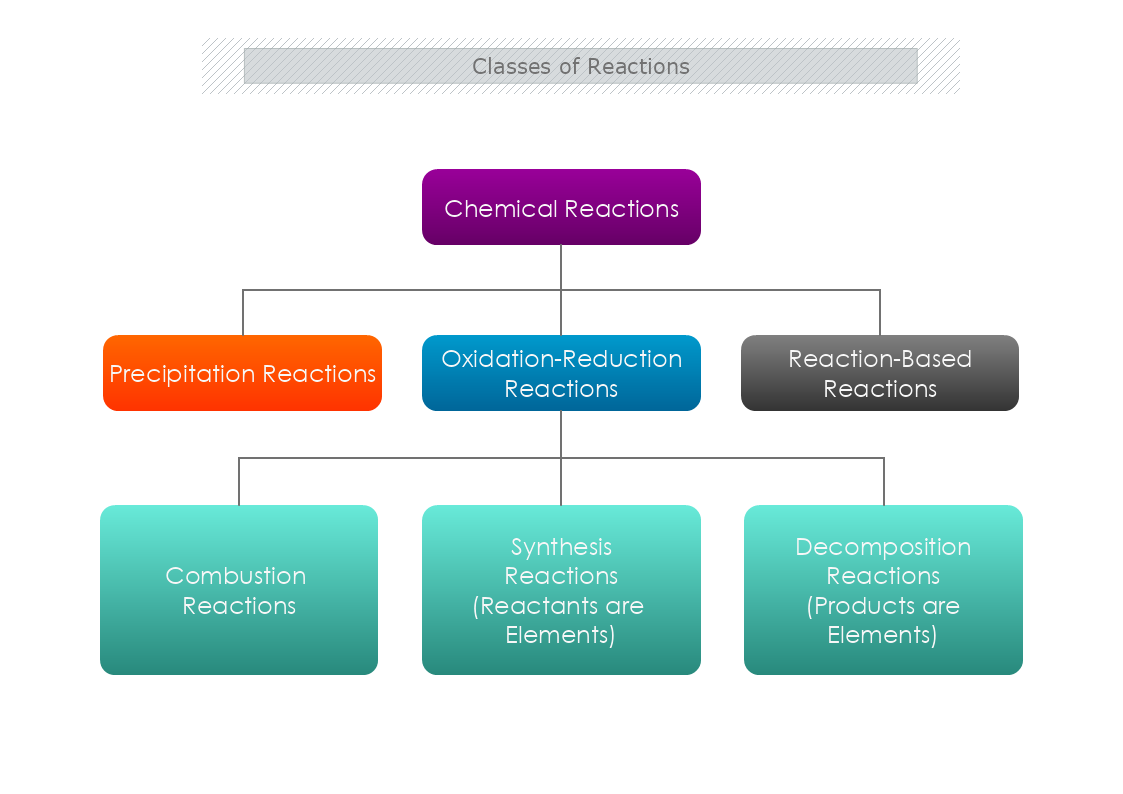
How to classify chemical reactions?
Precipitation reactions- the chemical reaction occurs in aqueous solutions where two ions bond together to form insoluble salts. The formation of these soluble salts are precipitates that are the products of it. Also, they can be single displacement reactions or double displacement reactions.
In a single replacement reaction, only one ionic compound dissociates. Whereas, in a double displacement reaction, both ionic reactants dissociate in water and their ions bond with the respective cation or anion from the other reactant. The result is an insoluble product.
- KCl (aq.) + AgNO3 (aq.) → KNO3 (aq.) + AgCl (s/ppt.)
In this chemical reaction between potassium chloride (KCl) and silver nitrate (AgNO3), silver chloride (AgCl) is precipitated out, and potassium nitrate (KNO3) remains in the solution.
Examples of precipitation reactions in real life
- Precipitation in water pipes.
- Formation of kidney stone.
- Formation of the immune complex.
Oxidation-reduction reactions- chemical reactions occur when one or more electrons are transferred from a reducing agent to an oxidizing agent. Also named redox reactions, the simple form includes the reaction of an element with oxygen. For example, magnesium burns in oxygen to form magnesium oxide (MgO). The product is an ionic compound, made up of Mg2+ and O2− ions.
- Fe2O3(s)+2Al(s)→Al2O3(s)+2Fe(l)
In this chemical reaction iron atoms in ferric oxide lose or give up O atoms to Al atoms, producing Al2O3.
Importance of redox reactions in our lives
- Photosynthesis
- Respiration
- Combustion
- Corrosion
Also, there are Reaction-Based Reactions.
How to make a Classes of Reactions template in MyDraw?
- Edit the ready-made template or open a “Blank Drawing” file to create your own.
- When you open a document in MyDraw a set of Basic and Connector shapes are loaded. You can choose from them and make the template.
- In this example, the template is made as an Organizational chart. You can also edit one of these templates to make yours.
- Also, from Library Gallery> ClipArt, you can select and browse through a variety of shapes you would like to use.
- Once you have checked and marked the shapes, they will be loaded on the left side of your drawing panel.
- Drag and drop the shapes you would like to use into the drawing.
- Use the connector tools to arrange your diagram.
- To add fill, you can edit the Geometry Fill and Stroke from the Ribbon.
- In the Ribbon, you can select the Design tab to choose from a variety of shape styles and theme colors.
- Save the document in one of MyDraw’s native formats or export it in a preferred file format (PDF, SVG, EMF, VSDX, etc.).
- You can also export the document as a raster image.
